In industries where sterilization is critical, such as healthcare, pharmaceuticals, and laboratories, ensuring the effectiveness of sterilization processes is non-negotiable. One of the most reliable methods for verifying sterilization efficiency is autoclave temperature mapping. This technique helps organizations maintain compliance with safety standards, protect products, and guarantee consistent sterilization outcomes.
What is Autoclave Temperature Mapping?
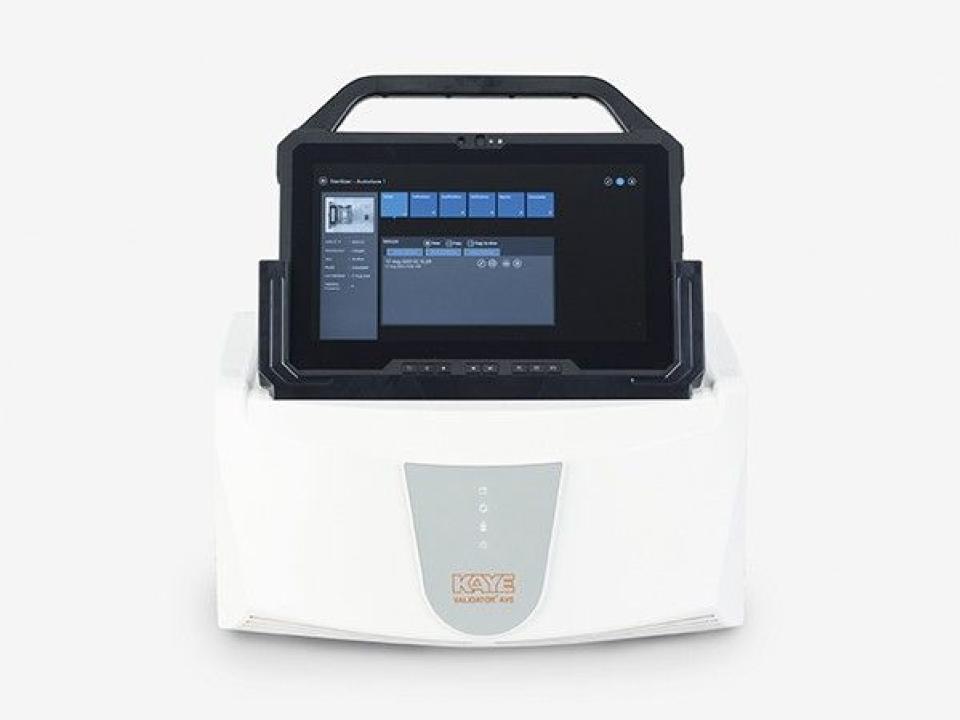
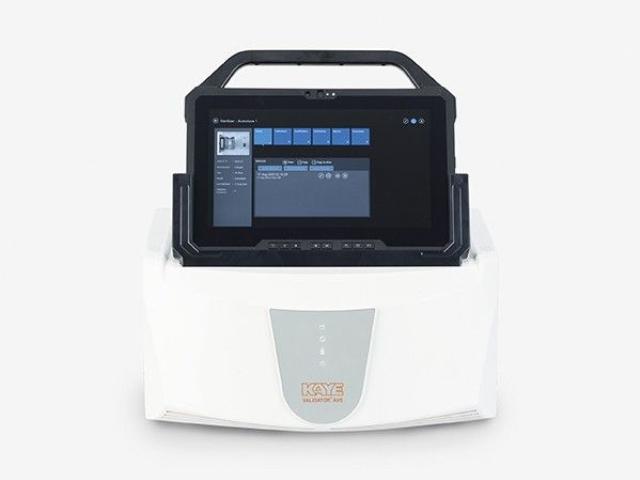
Autoclave temperature mapping is the systematic process of measuring and recording the temperature at various locations within an autoclave during a sterilization cycle. Autoclaves rely on high-pressure saturated steam to eliminate bacteria, viruses, and spores from instruments or products. However, temperature distribution inside the chamber may not always be uniform. Hot and cold spots can occur due to chamber design, loading patterns, or steam flow. Temperature mapping identifies these variations, ensuring that all items receive proper sterilization.
The Importance of Temperature Mapping
Without proper autoclave temperature mapping, there is no way to verify that sterilization conditions meet regulatory and safety standards. Medical instruments, laboratory equipment, and pharmaceutical products that are inadequately sterilized pose significant risks, including contamination, infection, and compromised research results. Conducting temperature mapping allows organizations to:
- Validate Sterilization Cycles: Mapping ensures that every point in the autoclave reaches the required temperature for the correct duration.
- Enhance Safety Compliance: Regulatory bodies, such as the FDA and ISO, often require documented validation of sterilization processes.
- Autoclave temperature mapping provides the necessary evidence.
- Optimize Load Placement: By identifying temperature inconsistencies, staff can adjust loading patterns to ensure uniform sterilization.
- Maintain Equipment Performance: Regular mapping helps detect autoclave malfunctions or areas that need maintenance before they compromise sterilization quality.
How Autoclave Temperature Mapping is Conducted
The process begins with placing multiple calibrated temperature sensors or data loggers throughout the autoclave chamber. These sensors record temperature readings during a standard sterilization cycle. Data collected is then analyzed to identify the warmest and coldest spots in the chamber. Special software is often used to generate detailed reports, including temperature charts and graphs, which help validate the autoclave’s performance.
A complete autoclave temperature mapping study typically involves:
- Initial Qualification: Mapping a new autoclave before it is put into regular use.
- Routine Verification: Periodic mapping to ensure consistent performance over time.
- Post-Repair Validation: Conducting mapping after maintenance or modifications to the autoclave.
Benefits of Implementing Autoclave Temperature Mapping
Organizations that implement autoclave temperature mapping reap multiple benefits. First, it ensures the sterilization process is both effective and reproducible. Second, it enhances patient and product safety by reducing the risk of contamination. Third, it helps maintain compliance with stringent regulatory standards, avoiding costly audits or recalls. Finally, it prolongs the life of autoclave equipment by identifying areas that require maintenance before major issues occur.
Conclusion
Autoclave temperature mapping is an essential practice for any facility that relies on sterilization to maintain safety and quality. By systematically measuring temperature across the autoclave chamber, organizations can ensure that every item is effectively sterilized, maintain compliance with industry regulations, and optimize autoclave performance. Whether you are in healthcare, pharmaceuticals, or laboratory research, implementing regular autoclave temperature mapping is a critical step toward achieving consistent sterilization results and safeguarding health and safety.