Introduction:
The global pharmaceutical market, valued at $1.48 trillion in 2024 (Statista), is facing increasing threats from counterfeit drugs, costing the industry over $200 billion annually (WHO). With supply chains stretching across continents, ensuring authenticity and traceability is critical. Pharma tokenization, built on the principles of asset tokenization, is emerging as a game-changer. This technology creates a unique, verifiable digital token for every product unit, enabling real-time tracking, fraud prevention, and compliance with regulatory requirements. By 2030, experts predict blockchain-based tokenization could save the pharmaceutical sector billions in counterfeit prevention and logistics optimization.
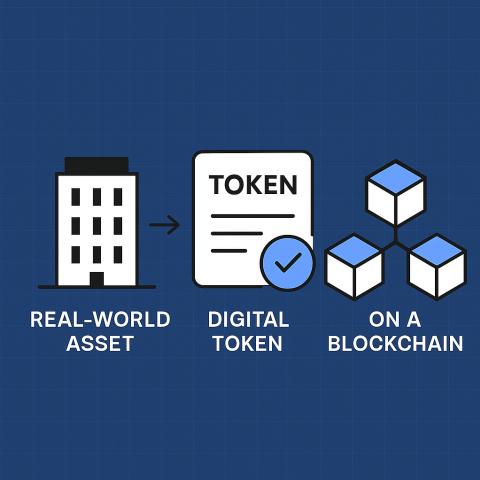
What is Asset Tokenization?
Asset tokenization is the process of converting ownership or rights to an asset into a digital token stored securely on a blockchain. Each token represents a unique, verifiable record that cannot be altered without consensus.
In the pharmaceutical sector, asset tokenization means that every vial, pill pack, or shipment can be represented digitally, allowing companies to track their journey from manufacturing to the patient’s hands.
Why It Matters:
Reduces fraud and counterfeiting
Enhances transparency for regulators and consumers
Speeds up recalls and quality assurance checks
Understanding Pharma Tokenization
Pharma tokenization applies asset tokenization directly to the pharmaceutical supply chain. Instead of tracking inventory through spreadsheets and barcodes alone, companies use blockchain to create tamper-proof digital twins of each product.
How It Works:
Token Creation – At the manufacturing stage, a unique token is generated for each product batch or unit.
Blockchain Registration – The token stores manufacturing details, batch numbers, expiry dates, and shipment logs.
Real-Time Tracking – Distributors, pharmacies, and hospitals can verify product authenticity instantly
Immutable Records – Data cannot be altered without detection, ensuring complete supply chain integrity.
The Need for Pharma Tokenization
The World Health Organization reports that 1 in 10 medical products in low- and middle-income countries is substandard or falsified. In some regions, counterfeit drugs account for up to 30% of the market.
Pharma tokenization directly addresses these challenges by:
-
Preventing counterfeit infiltration into supply chains
-
Ensuring regulatory compliance with standards like DSCSA (U.S.) and FMD (EU)
-
Enabling faster recalls in case of contamination or safety issues
Benefits of Pharma Tokenization
-
Enhanced Security
Each product token is cryptographically secured, making it nearly impossible to forge. -
Improved Traceability
From factory to pharmacy, every handoff is recorded in real time. -
Regulatory Confidence
Regulators can audit supply chains without relying solely on company-reported data.Cost Savings
Reduces losses from fraud, delays, and recall inefficiencies — potentially saving billions annually.
Real-World Examples
-
Moderna & IBM Blockchain: Explored blockchain-based tracking for COVID-19 vaccine distribution.
-
Chronicled: Developed the MediLedger Network, a blockchain platform for pharmaceutical verification.
-
SAP Pharma Blockchain: Helping global drug manufacturers tokenize and verify shipments.
Future Outlook
By 2027, blockchain in healthcare is expected to reach $5.61 billion (Allied Market Research), with pharma tokenization as a major contributor. The integration of AI-powered analytics with tokenized supply chains will make drug distribution even more secure and efficient.
FAQs
Q1: Is Pharma Tokenization the same as serialization?
No — serialization assigns unique identifiers, but pharma tokenization goes further by recording them on a blockchain, making the data immutable.
Q2: Can small pharma companies afford tokenization?
Yes. As blockchain infrastructure becomes cheaper, even mid-sized and small manufacturers can implement tokenized tracking.
Q3: Does Pharma Tokenization work with existing supply chain software?
Yes, most solutions integrate with ERP and logistics platforms.
Final Takeaway
Pharma tokenization is not just a tech trend — it’s a necessity for a world battling counterfeit drugs and complex global supply chains. By leveraging asset tokenization, the pharmaceutical industry can ensure authenticity, compliance, and efficiency from production to patient delivery.
In the coming years, as regulations tighten and patient safety becomes even more critical, pharma tokenization will likely become the gold standard for securing drug supply chains.